Blood Glucose Meter Technical Requirements¶
Medical Device Product Technical Requirements Number:
Version: v1.0
Document Code: SOP-13
Effective Date: December 17, 2025
1. Product Model/Specifications and Classification¶
1.1 Product Model/Specifications¶
Model: GDHFAD Meter Plus and Meter Mini Blood Glucose Meters
Model Designation: "GDH" represents that the product uses Glucose Dehydrogenase as the core detection enzyme, "FAD" represents the specific subtype of the enzyme (such as Flavin Adenine Dinucleotide-dependent type, ensuring reaction specificity), no sub-specifications, all products of this model have consistent core performance and structural composition, only accessory configurations (such as whether spare batteries are included) may vary slightly depending on the sales package.
1.1.1 Software Name¶
GDHFAD Blood Glucose Meter Blood Glucose Detection and Data Processing Internal Working Program
1.1.2 Release Version: V1¶
1.1.3 Complete Version: V1.0.0.0¶
1.1.4 Software Naming Convention¶
V X.Y.Z.B
Where: - V - Version abbreviation - X - Major version number, representing major enhancement software updates; when function modules or algorithms have significant changes, such as adding multiple function modules or overall architecture changes, this major version number increases by 1 - Y - Minor version number: representing minor enhancement software updates; when functions have certain additions or changes, such as adding operation function permission control, adding device control instructions, this minor version number increases by 1 - Z - Stage version number: representing corrective software updates; generally for software bug fixes or minor changes, such as interface image modifications, interface function description text additions or deletions, etc., stage versions should be released frequently, with no time interval limit, a stage version can be released for fixing one bug, each time a stage version is released, this stage version number increases by 1 - B - Build version number: this version number is used to mark the number of times the current version generates executable programs
2. Performance Requirements¶
2.1 Appearance and Structure¶
2.1.1 The blood glucose meter product shape should be upright, the surface should be bright and clean, with no sharp edges, burrs, damage or deformation¶
2.1.2 Text and markings on the blood glucose meter control panel should be accurate, clear, and firmly attached¶
2.1.3 Display characters on the screen should have no garbled characters, wrong codes or missing strokes¶
2.1.4 Test strip slot port should be smooth with no burrs on edges, ensuring GDHFAD special blood glucose test strips insert smoothly with good contact¶
2.1.5 Function buttons (power, query, eject strip, etc.) should press flexibly with reliable rebound, battery compartment and housing fasteners should not be loose¶
2.2 Blood Glucose Concentration Display Range¶
Blood glucose meter blood glucose concentration display range is 0.6mmol/L~33.3mmol/L (20mg/dL~600mg/dL), when exceeding the range, the display screen should clearly show "LO" (<0.6mmol/L) or "HI" (>33.3mmol/L).
2.3 Blood Glucose Meter Measurement Range¶
2.3.1 Blood glucose meter measurement range is 2.2mmol/L~27.8mmol/L (40mg/dL~500 mg/dL), linear correlation coefficient r not less than 0.95¶
2.3.2 Blood Glucose Meter Repeatability¶
Blood glucose meter repeated measurement results precision should meet the requirements of Table 1:
Table 1 Repeatability Requirements
| Test Range | Precision |
|---|---|
| 2.2mmol/L ~ 5.5mmol/L(40mg/dL ~ 100mg/dL) | SD<0.42 mmol/L(<7.7 mg/dL) |
| 5.6mmol/L ~ 27.8mmol/L(100mg/dL ~ 500mg/dL) | CV<7.5% |
2.3.3 Blood Glucose Meter Accuracy¶
Blood glucose meter accuracy should meet one of the following requirements:
a) 95% of blood glucose meter measurement result deviations should meet the requirements of Table 2; b) Blood glucose meter glucose recovery rate is 80%~120%.
Table 2 Accuracy Requirements
| Test Range | Allowable Deviation |
|---|---|
| 0.6mmol/L ~ 5.5mmol/L(11mg/dL ~ 100mg/dL) | Not exceeding ±0.83 mmol/L(±15 mg/dL) |
| 5.5mmol/L ~ 33.3mmol/L (100 mg/dL~ 600mg/dL) | Not exceeding ±16% |
2.3.4 95% of blood glucose meter quality control material measurement results should be within the quality control range.¶
2.3.5 Under changing environmental conditions (temperature 5℃~45℃, humidity ≤90% RH), the above error requirements still need to be met;¶
2.3.6 Blood glucose meter does not provide readings outside the display range, displaying "LO" (<0.6mmol/L) or "HI" (>33.3mmol/L).¶
2.4 Drop Resistance¶
From a vertical distance of 1m height, in three postures of "front, side, test strip slot end" freely drop onto a hard surface (density >700kg/m³ hard wood block), after dropping the instrument functions normally, no component damage, and accuracy tested according to 2.3.1 requirements still meets standards.
2.5 Indication Unit¶
2.5.1 Resolution¶
Display screen blood glucose value resolution is 0.1mmol/L (or 1mg/dL, depending on unit settings).
2.5.2 Display¶
Blood glucose value display character height ≥4mm, ensuring clear reading for people with different vision levels;
2.5.3 Prompt Function¶
- Low battery prompt: When supply voltage ≤2.5±0.2V, battery symbol flashes accompanied by "beep" warning sound;
- Environmental abnormality prompt: When ambient temperature <10℃ or >40℃, display screen shows corresponding error code (such as "E-2" indicating temperature abnormality), and emits warning sound;
- Test strip error prompt: When inserting non-GDHFAD special strips, expired strips or improperly inserted strips, displays "E-3" "E-4" and other error codes;
2.5.4 Mode¶
Has "blood glucose detection mode" and "quality control detection mode" (used to verify GDHFAD blood glucose test strip validity).
2.6 Product Cleaning and Disinfection¶
- Use 75% alcohol cotton pads to wipe blood glucose meter housing, display screen, test strip slot exterior (not internal circuits), after cleaning and disinfection:
- Accuracy still meets 2.3 requirements;
- Housing text and markings have no blurring or peeling;
- Prohibit use of gasoline, strong acids and alkalis and other corrosive cleaners.
2.7 Normal Working Status¶
After inserting GDHFAD special test strips, the instrument automatically powers on for self-test, emits a "beep" prompt, when the blood drop symbol flashes, absorb blood sample (about 3μL), complete detection within 5 seconds and display blood glucose value, without freezing or crashing.
2.8 Self-test Function¶
After power on, LCD display screen fully lights up (all symbols light up), lasting ≥0.1 seconds, after self-test with no abnormalities enters "blood drop waiting for detection" state; if self-test is abnormal (such as internal circuit failure), displays corresponding error code (such as "E-6").
2.9 Automatic Shutdown Function¶
After detection is complete with no operation, or blood drop waiting 3 minutes with no blood sample absorption, or standby 1 minute with no operation, the instrument automatically shuts down, reducing power consumption.
2.10 Memory Function¶
Can store the most recent 500 groups (or adjust according to design requirements) of blood glucose test data, including measurement time and blood glucose value, supports query in chronological order, new data storage automatically overwrites the earliest data.
2.11 Electrical Safety Evaluation¶
Complies with GB9706.1-2020 "Medical Electrical Equipment Part 1: Safety General Requirements", electric shock protection type is "internal power supply equipment", electric shock protection degree is "Type B applied part".
2.12 Electromagnetic Compatibility¶
Complies with YY 0505-2012 "Medical Electrical Equipment Part 1-2: Safety General Requirements Collateral Standard: Electromagnetic Compatibility Requirements and Tests", in common radio frequency environments (such as mobile phones, microwave ovens), detection results are not interfered with.
2.13 Environmental Test Requirements¶
- Climatic environment test: Complies with GB/T 14710-2009 Group II requirements, such as low temperature storage (-20℃, 4h), high temperature storage (55℃, 4h), humid heat storage (40℃, 93% RH, 48h) after which the instrument functions normally with accuracy meeting standards;
- Mechanical environment test: Complies with GB/T 14710-2009 Group II vibration and collision requirements;
- Transport test: Under normal packaging conditions, after road/rail transport simulation test, all performance (except 2.11, 2.12) meets requirements;
- Power supply voltage adaptation capability: Using matching battery (such as 1 button battery, DC 3V), when voltage fluctuates in the range of 2.7V~3.3V, accuracy still meets 2.3 requirements.
Specific experimental requirements and test items are shown in Table 3 below.
Table 3 Environmental Test Items, Test Requirements and Test Items
| Test Item | Test Requirements | Test Items | Remarks | |||||
|---|---|---|---|---|---|---|---|---|
| In-chamber test time h | In-chamber duration h | Recovery time h | Intermediate detection | Final detection | Power voltage adaptation capability test | |||
| 2.7 V | 3.15 V | |||||||
| Normal temperature test | - | - | - | - | Full performance | - | - | - |
| Rated working low temperature test 16℃ | 1 | - | - | - | 2.2, 2.3.1 | 2.2, 2.3.1 | - | Power on |
| Low temperature storage test -20℃ | 4 | - | 4 | - | - | - | - | |
| Rated working high temperature test 35℃ | 1 | - | - | 2.2, 2.3.1 | - | 2.2, 2.3.1 | Power on | |
| Operation test | 4 | - | - | - | - | 2.2, 2.3.1 | Power on | |
| High temperature storage test 55℃ | 4 | - | 4 | - | - | - | - | |
| Rated working humid heat test 35℃ 85%RH | 4 | - | - | - | - | - | Power on | |
| Humid heat storage test 40℃ 93%RH | 48 | - | 24 | - | - | - | - | - |
| Vibration collision test | One test direction, normal working position | - | 2.1 | - | - | - | ||
| Transport test | Normal packaging state | - | Full performance (except 2.11, 2.12) | - | - | - |
3. Test Methods¶
Normal Working Conditions
a) Environmental blood glucose concentration: 5℃~45℃; b) Relative humidity: ≤90%; c) Atmospheric pressure: 70kPa~106kPa; d) Power supply conditions: Internal DC power supply: DC 3.0V (1 button battery).
Test conditions: Testing should be conducted in accordance with test conditions specified in GB/T 21417.1-2008 5.1 and 5.2.
3.1 Appearance and Structure Test¶
Visual observation and tactile inspection, results should meet 2.1 requirements.
3.2 Blood Glucose Concentration Display Range Test¶
Test with standard glucose solution at concentrations of 0.5mmol/L ("LO" critical point) and 33.3mmol/L ("HI" critical point), confirm the instrument displays "LO" "HI"; Record the blood glucose concentration reading displayed by this device, results should meet 2.2 requirements.
3.3 Blood Glucose Meter Measurement Range¶
3.3.1 Blood Glucose Meter Measurement Range Test¶
Referring to "GB/T 19634-2005 In Vitro Diagnostic Testing Systems Self-Testing Blood Glucose Monitoring Systems General Technical Conditions", take venous whole blood samples with heparin anticoagulant added, finally making 7 samples with glucose concentrations within the range of Table 5, with the 1st and 7th samples close to 2.2 mmol/L and 27.8 mmol/L respectively. Divide the prepared samples into two equal parts, one part uses an analyzer for glucose measurement, the other part uses two blood glucose meters and the same batch number blood glucose test strips to measure 10 times each. For each measurement sample, the analyzer measurement value is xi, the blood glucose meter measurement value is yi, calculate the correlation coefficient of the two sets of data according to formula (1). Results should meet 2.3.1 requirements.
Where:
- \(r\) — correlation coefficient
- \(n\) — number of samples
- \(x_i\) — analyzer measurement value for the i-th sample
- \(y_i\) — blood glucose meter measurement value for the i-th sample
- \(\bar{x}\) — average value of analyzer measurements
- \(\bar{y}\) — average value of blood glucose meter measurements
3.3.2 Blood Glucose Meter Repeatability Test¶
Collect fasting fresh venous whole blood from blood sample providers with hematocrit of 0.35L/L to 0.50L/L (35% to 50%), inject into test tubes with heparin or EDTA anticoagulant, avoiding hemolysis. Place samples at room temperature (23±5)℃ to equilibrate, bringing sample temperature to room temperature ±2℃, as the temperature at test start. Should use 5 venous whole blood samples with blood glucose concentration ranges falling in Table 4 for repeatability testing. Each sample should be gently inverted to mix thoroughly before testing. The concentration of each sample should be determined using an analyzer. Then repeat testing 20 times on two blood glucose meters according to the manufacturer's routine measurement procedure. Calculate the standard deviation (SD) and coefficient of variation (CV) of results, results should meet 2.3.2 requirements.
Table 4 Blood Glucose Concentration Range for Repeatability Testing
| Blood Glucose Concentration [mmol/L (mg/dL)] |
|---|
| 2.2 ~ 2.8(40 ~ 50) |
| 2.9 ~ 6.1(51 ~ 110) |
| 6.2 ~ 8.3(111 ~ 150) |
| 8.4 ~ 13.9(151 ~ 250) |
| 14.0 ~ 22.2(251 ~ 400) |
Note: The glucose concentration in venous blood samples can be adjusted with equal parts glucose solution prepared with 0.9% saline. The degree of dilution should not affect the sample matrix. Sufficient preservatives that meet manufacturer recommendations and do not interfere with blood glucose detection (such as: maleimide, fluoride, or iodoacetate solution) should be added to samples to avoid glycolysis. To obtain low glucose concentration blood samples, anticoagulated blood samples can be left overnight to allow glucose concentration to drop to the required level.
3.3.3 Blood Glucose Meter Accuracy Test¶
Testing venous blood samples (replacing capillary blood samples).
3.3.3.1 Comparison Test¶
Use 50 venous whole blood samples with blood glucose concentration ranges falling in Table 5. Before testing with each sample, gently invert to mix thoroughly. Each venous blood sample is divided into 3 parts, the 1st part is tested with the 1st blood glucose meter, the 2nd part is tested with the 2nd blood glucose meter, testing should be conducted according to the manufacturer's routine measurement procedure; the 3rd part is centrifuged at high speed, after removing plasma, an analyzer is used for blood glucose testing. The difference between the venous whole blood results \(\bar{y_i}\) tested by each blood glucose meter and the results \(\bar{x_i}\) tested by the analyzer is the accuracy deviation, results should meet clause a of 2.3.3 requirements.
Accuracy calculation formula:
Absolute deviation:
Relative deviation:
Where:
- \(\bar{y_i}\) — venous whole blood results tested by blood glucose meter
- \(\bar{x_i}\) — results tested by analyzer
Table 5 Blood Glucose Concentration Range for Accuracy Testing
| Sample Quantity | Blood Glucose Concentration [mmol/L (mg/dL)] |
|---|---|
| 2 | <2.8 (<50) |
| 8 | 2.8~4.3(50~80) |
| 10 | 4.4~6.7(81~120) |
| 15 | 6.7~11.1(121~200) |
| 8 | 11.2~16.6(201~300) |
| 5 | 16.7~22.2(301~400) |
| 2 | >22.2(>400) |
Note: Samples with blood glucose concentration in the range of 2.8mmol/L~22.2mmol/L (50mg/dL~400mg/dL) should be obtained from original samples.
Extreme concentration samples at both ends can be obtained by the following methods: Collect blood samples in test tubes with anticoagulant, incubate them in a 37℃ incubator overnight to cause blood glucose glycolysis, obtaining samples with blood glucose concentration < 2.8mmol/L; Collect blood samples in test tubes with anticoagulant, then add appropriate glucose, obtaining samples with blood glucose concentration > 22.2mmol/L.
3.3.3.2 Recovery Test¶
Take venous whole blood sample S1 with heparin anticoagulant added, sufficient preservatives that meet manufacturer recommendations and do not interfere with blood glucose detection can be added to samples to avoid glycolysis. Accurately pipette 7 equal portions of S1 (volume V1), add V2 (S2), V3 (S3), V4 (S4), V5 (S5), V6 (S6), V7 (S7) standard glucose saline solutions of known concentration to 6 of them respectively, finally making the glucose concentrations of the 7 samples fall within the range of Table 5 respectively. Each sample is gently inverted to mix thoroughly, test the 7 samples 10 times each using 2 blood glucose meters and the same batch number blood glucose test strips, the average of 10 measurements are recorded as C1, C2, C3, C4, C5, C6, C7 respectively. Calculate the glucose recovery rate of the blood glucose meter and blood glucose test strips according to the following formula, test results should meet clause b requirements of 2.3.3.
Where: - \(R\) — recovery rate - \(n=1,2,3,4,5,6\) - \(i=2,3,4,5,6,7\) - \(V_i\) — volume of glucose saline solution added between samples \(S\) and \(S_i\) - \(V_1\) — volume of sample \(S_1\) - \(C_i\) — measured concentration of sample \(S_i\) - \(C_1\) — measured concentration of sample \(S_1\) - \(C_s\) — concentration of prepared glucose solution
3.3.4 Blood Glucose Meter Quality Control Material¶
With the same blood glucose meter and same batch blood glucose test strips, repeatedly measure quality control material concentration 20 times, should meet 2.3.4 requirements.
3.3.5 Under changing environmental conditions (temperature 5℃~45℃, humidity ≤90% RH), repeat the above tests, test error requirements meet 2.3.5 requirements;¶
3.3.6 Test with standard glucose solution at concentrations of 0.5mmol/L ("LO" critical point) and 33.3mmol/L ("HI" critical point), confirm the instrument displays "LO" "HI" without providing readings; test results should meet 2.3.6 requirements.¶
3.4 Drop Resistance Test¶
Complete 3 drops according to 2.4 requirements, after dropping test 3 times with 5.0mmol/L standard glucose solution, error meets 2.3 requirements, and buttons, display screen, test strip slot functions are normal. After drop test, test according to 3.3.1, results should meet 2.4 requirements.
3.5 Indication Unit Test¶
3.5.1 Resolution¶
Visual observation of display screen values, confirm resolution and character height, results should meet 2.5.1.
3.5.2 Display¶
Actually operate to switch "detection mode" "quality control mode", confirm functions are normal, results should meet 2.5.2.
3.5.3 Prompt/Alarm Function¶
-
Blood glucose concentration exceeding limit prompt test: Under laboratory reference conditions (temperature 23±2℃, relative humidity 50%±10% RH, atmospheric pressure 70kPa~106kPa), equilibrate GDHFAD blood glucose meter and matching standard glucose solution (concentration 0.5mmol/L, corresponding to "LO" critical value; 33.3mmol/L, corresponding to "HI" critical value) for at least 30 minutes. Take 0.5mmol/L standard glucose solution, absorb into test strip according to normal detection procedure, repeat measurement 3 times, observe instrument display, should accurately display "LO" and corresponding prompt (such as "blood glucose too low"), meeting 2.5.3 requirements; Take 33.3mmol/L standard glucose solution, repeat measurement 3 times using same method, observe instrument display, should accurately display "HI" and corresponding prompt (such as "blood glucose too high"), meeting 2.5.3 requirements.
-
Environmental temperature and humidity abnormality prompt test: a) Before testing, stabilize GDHFAD blood glucose meter under laboratory reference conditions for 30 minutes, then place in the following environments respectively: b) Low temperature environment: <16±1℃ (such as 15℃), relative humidity 50%±10% RH, after placing for 30 minutes, insert test strip and attempt detection, instrument should display temperature abnormality code (such as "E-2") and emit warning sound, meeting 2.5.3c requirements; c) High temperature environment: >35±1℃ (such as 36℃), relative humidity 50%±10% RH, after placing for 30 minutes, detect using same method, instrument should display temperature abnormality code (such as "E-2") and emit warning sound, meeting 2.5.3d requirements.
3.5.4 Low Voltage Prompt Function Test¶
Use DC regulated power supply to replace the blood glucose meter's internal DC power supply. Lower voltage to 2.5V±0.2V, blood glucose meter should display a low battery indication or warning, or display starts to dim, results should meet 2.5.4 requirements.
3.5.5 Mode¶
Actually operate GDHFAD blood glucose meter, switch modes according to manual steps:
Switch from default "blood glucose detection mode" to "quality control detection mode" (used to verify test strip validity), observe whether display screen shows "quality control mode" identifier;
In "quality control mode" insert test strip, absorb quality control solution, confirm instrument can normally complete quality control detection and display results, switching process has no freezing, functions meet 2.5.5 requirements.
3.6 Product Cleaning and Disinfection¶
a) Test GDHFAD blood glucose meter according to the following steps, simulating daily cleaning scenarios: b) Prepare 75% medical alcohol and distilled water two cleaning reagents, use soft cotton cloth to absorb corresponding reagents respectively; c) Use cotton cloth with alcohol absorbed to gently wipe instrument housing, display screen, test strip slot exterior (avoiding liquid penetration into internal circuits), air dry naturally after each wipe, repeat operation 20 times; d) Use same method with cotton cloth with distilled water absorbed to repeat cleaning 20 times; e) After cleaning inspection: f) Visually inspect instrument surface, should be bright and clean, no reagent residue, housing corrosion marks; g) Check housing text and markings (such as blood drop symbol, operation prompts), should have no blurring or peeling; h) According to 3.3.1 (accuracy test) method, measure 3 times with 5.0mmol/L standard glucose solution, error should meet 2.6 requirements (i.e. consistent with accuracy before cleaning, no obvious deviation).
3.7 Normal Working Status Test¶
a) Prepare GDHFAD blood glucose meter, matching new test strips, 5.0mmol/L standard glucose solution, equilibrate under laboratory reference conditions for 30 minutes; b) Insert test strip, instrument automatically powers on for self-test, after blood drop symbol flashes, bring test strip sampling end in contact with standard glucose solution, confirm blood sample is smoothly absorbed; c) Observe instrument operation procedure: should complete detection within 5 seconds and display blood glucose value (error should be within ±0.83mmol/L), without crashing, freezing, display abnormality, meeting 2.7 requirements.
3.8 Self-test Function Test¶
a) Press GDHFAD blood glucose meter power button to turn on, observe display screen status; b) Instrument should first enter self-test mode, LCD display screen fully lights up (all symbols and numbers light up, such as "888" "blood drop symbol" "battery symbol"), lasting ≥0.1 seconds; c) After self-test completes, display screen should switch to "blood drop waiting for detection" state (displaying blood drop symbol or "---"), no error code prompts, meeting 2.8 requirements; if self-test is abnormal (such as internal circuit failure), should display corresponding error code (such as "E-6"), prompt function is normal.
3.9 Automatic Shutdown Function Test¶
a) After power on without inserting test strip, maintain standby state, time observation: within 1 minute without any operation, instrument should automatically shut down, meeting 2.9 requirements; b) Insert test strip, after blood drop symbol flashes without absorbing blood sample, time observation: within 3 minutes without operation, instrument should automatically shut down, meeting 2.9 requirements; c) After completing one normal detection (displaying blood glucose value) without removing test strip, time observation: within 3 minutes without operation, instrument should automatically shut down, meeting 2.9 requirements.
3.10 Memory Function¶
a) Prepare 10 groups of standard glucose solutions with different concentrations (such as 1.0mmol/L, 3.0mmol/L, 5.0mmol/L…20.0mmol/L), complete detection in order from low to high concentration, record instrument display storage sequence number after each group detection; b) After detection completes, enter "historical data query" mode according to manual steps, view stored data in sequence: should be able to completely display 10 groups of blood glucose values and corresponding detection time (if instrument has time recording function), no data loss or disorder; c) Conduct 1 more new detection (group 11), query historical data: the earliest stored group 1 data should be automatically overwritten, only retaining the last 10 groups of data, meeting 2.10 requirements (Note: if instrument design memory capacity is other quantity, adjust test group number according to corresponding capacity).
3.11 Electrical Safety Evaluation Test¶
a) Test according to methods specified in GB9706.1-2020 "Medical Electrical Equipment Part 1: Safety General Requirements", focusing on detecting: b) Electric shock protection: Check equipment housing insulation performance, grounding continuity (if any), ensure no electric leakage risk; c) Power supply voltage adaptation: Within 2.7V~3.3V voltage range, instrument can still work normally, accuracy meets requirements; d) Overload protection: Simulate battery short circuit scenario, instrument should have protection mechanism, no damage or fire risk; e) Test results should meet 2.11 requirements.
3.12 Electromagnetic Compatibility¶
a) Test according to methods specified in YY 0505-2012 "Medical Electrical Equipment Part 1-2: Safety General Requirements Collateral Standard: Electromagnetic Compatibility Requirements and Tests", including: b) Electromagnetic emission test: Detect radio frequency radiation and conducted emission when instrument is working, should meet "Group 1 Class B" limits; c) Electromagnetic immunity test: Simulate electrostatic discharge (±8kV air discharge), radio frequency electromagnetic field (3V/m), power frequency magnetic field (3A/m) and other interference scenarios, instrument detection result error should still meet 2.3 (accuracy) requirements, without crashing or display abnormality, meeting 2.12 requirements.
3.13 Environmental Test¶
GDHFAD blood glucose meter environmental test according to GB/T 14710-2009 "Medical Electrical Equipment Environmental Requirements and Test Methods"
4. Terminology¶
None
Appendix A¶
A.1 Product Characteristics¶
A 1.1 Classification by electric shock protection type: Internal power supply;
A 1.2 Classification by degree of electric shock protection: Type B applied part;
A 1.3 Classification by degree of protection against liquid ingress: Not protected against liquid ingress;
A 1.4 Classification by safety degree when used with flammable anesthetic gas mixed with air or flammable anesthetic gas mixed with oxygen or nitrous oxide: Not AP/APG equipment;
A 1.5 Classification by operating mode: Continuous operation;
A 1.6 Equipment rated voltage: DC3V (1 button battery);
A 1.7 Whether equipment has applied part with protection against defibrillation discharge effects: Does not have;
A 1.8 Whether equipment has signal output or input part: None;
A 1.9 Permanently installed equipment or non-permanently installed equipment: Non-permanent;
A 1.10 Electromagnetic compatibility group classification: Classified as Group 1 Class B according to GB4824;
A 1.11 Electrical insulation diagram (below):
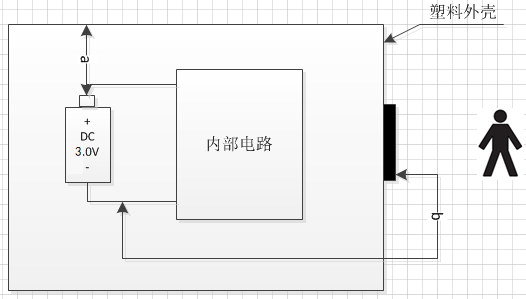
Insulation Diagram
Electrical Insulation Table
| Path | Insulation Type | Reference Voltage | Creepage Distance Required Value (mm) | Clearance Required Value (mm) | Creepage Distance Measured Value (mm) | Clearance Measured Value (mm) |
|---|---|---|---|---|---|---|
| Between live parts and unprotected grounded housing parts | DI/RI | d.c.3.0V | 3.4 | 1.6 | >4.4 | >2.1 |
| Between applied part and live parts | DI/RI | d.c.3.0V | 3.4 | 1.6 | >4.4 | >2.1 |
Document Control: - Prepared by: Quality Assurance Department - Reviewed by: Technical Department - Approved by: General Manager - Effective Date: December 17, 2025 - Version: v1.0